Crystallization is a physical change. Crystallization is the formation of solids from the liquid or gaseous phase. This technique includes obtaining the crystals of a soluble substance from a hot saturated solution and separating the soluble solid from the solution.
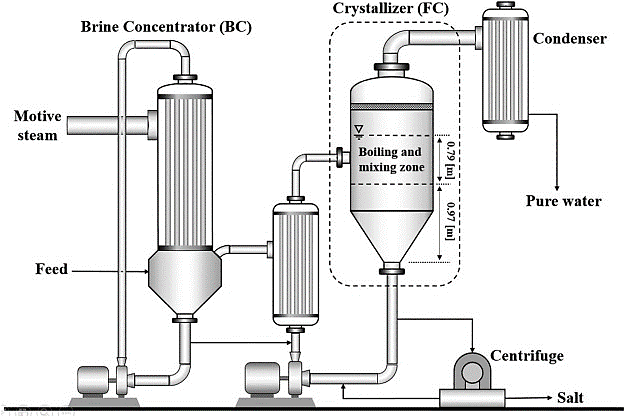
To concentrate feed into solid crystals and clean water, crystallizers are used. A progressively harder method for which crystallite sizes are formed from a liquid solution is known as crystallization. Crystallizers can remove liquid wastes completely, resulting in no liquid discharge (ZLD). The process continues and secondary crystallization are the two stages of crystallization. The formation of new crystals is referred to as primary nucleation. Secondary nucleation is the primary stage that results in the mass production of crystals. There are two types of crystallization processes: evaporative crystallization and cooling crystallization.
Planning a up coming technology photo voltaic crystallizer for genuine seawater brine procedure with zero liquid discharge
The salts are discharged by opening the flanged go over. The salt basket is perfect for blended salts with major crystal salts like sodium sulphate or sodium chloride which have been promptly dewatered.
a SEM picture on the crystals just after fully drying concentrated RO squander brine straight on wafer and also the corresponding EDS maps of b metallic aspects, c magnesium, d chloride, e sulfur, and file oxygen.
The location is safe. The https:// makes sure that you will be connecting into the official Web site Which any information you supply is encrypted and transmitted securely.
Slow cooling: It necessitates solvents with minimal boiling factors and modest solute solubility. A certain total of material is needed.
An average ZLD process is composed of a focus system, which concentrates the first source brine to in close proximity to saturation, as well as a crystallization system that fully eliminates all residual h2o from the brine to create solid salts11. Before a long time, the concentration system has actually been appreciably enhanced by different systems, including reverse osmosis (RO), electrodialysis, membrane distillation (MD), and mechanical vapor compression concentrator, and many others. Nevertheless, the development while in the crystallization system is extremely sluggish since all membrane centered desalination systems cannot perform with in close proximity to-saturation brines due to salt scaling and fouling7,eleven. The existing brine crystallization is especially achieved possibly by brine crystallizers or by evaporation ponds16.
A resin crystallizer allows flake or amorphous resin pellets to crystallize. To crystallize incredibly viscous and gradually crystallizing fill masses, a vertical ongoing cooling crystallizer (VCCC) is used. Other crystallizers tailored to distinct apps are also obtainable.
We hypothesize the followings being a feasible mechanism for forming the floor dense crust levels and inner pore filling crystals in Crystallizer Manufacturer Crystallizer For Zero Liquid Discharge System the case of your concentrated SWRO brines. Because the salt precipitates through water evaporation, NaCl, as by far the most ample species, precipitates dominantly although remarkably hydrous magnesium sulfate, as a small species, precipitates only from the hole House among the cubic NaCl crystals. Since the hydrous magnesium sulfate little by little fills the pore House among the NaCl crystals, at 1 position, a dense crust layer is shaped to the area, which renders the evaporation substrate not able to deliver drinking water to your outer floor and therefore drinking water evaporation stops.
S5a). In these situations, once the h2o evaporation costs had dropped to almost zero, these crystallizer surfaces appeared rather black, indicating the light absorption of such crystallizers was not significantly degraded because of the precipitated salt layer (Fig. S5b, c). As a result, it's thought that the variation in the light absorption was not the actual reason for the degraded drinking water evaporation prices because of the 3D crystallizer although managing the real brines.
Be aware: In Fig. 3c, an optional clear polycarbonate defend using a height of thirty mm was additional to the photo voltaic crystallizer in an effort to avoid salt crystal from creeping into the internal side of your crystallizer.
It really is uniquely present in all significant industrial segments with effective installations in these marketplaces with a all over the world foundation, generally as "
These brine crystals are frequently driven by steam but in some cases can use other technological innovation to recycle vapour to lessen Power use and working prices.
The salt layer when using photo voltaic crystallizer to treat a pure NaCl brine, b concentrated SWRO brine and c concentrated SWRO brine with NTA.
After the salt crust layer was immediately eliminated by a plastic spatula with none drinking water washing treatment method, the 3D solar crystallizer was able to provide an identical evaporation overall performance in the 2nd 24-h take a look at cycle (Fig. 4a), indicating the 3D photo voltaic crystallizer is often simply regenerated and reused without having noticeable transform in actual brine therapy general performance. It had been noticed that, if the photo voltaic mild was turned off during the operation, the evaporation rate dropped to all around 0.three kg m−two h−1 (Fig. 4a). Having said that, the surface area salt layer didn't show any visible change even following the photo voltaic crystallizer was kept in dark for 12 h (Fig.